Exciting The Brain To Stop Seizures?! - Jean-François Perrier, University of Copenhagen, Denmark
Surprising and exciting rare epilepsy (STXBP1) research discoveries have led to the enhancement of excitatory synapses as a proposed treatment - by Jean-François Perrier of the University of Copenhagen, Denmark.
Reported by Torie Robinson | Edited and produced by Pete Allen
Podcast
Jean-François Perrier’s research into the STXBP1 gene
-
00:00 Jean-François Perrier
Yes, so a common reason for epilepsy is lack of inhibition. If there is not enough inhibition, then there is some hyperactivity in some area of the brain, and then it can be sufficient to trigger seizures. And that's where we started. We wanted to understand why there is such a lack of inhibition00:17 Torie Robinson
Fellow homo sapiens! Now, welcome back to Epilepsy Sparks Insights.
Are you new to the epilepsies or epileptology in general? If you are…and actually if you aren’t as well(!), you might want to still have a pen and paper ready for this episode, because today we have scientist Jean Francois Perrier from the University of Copenhagen with us, who will be explaining his exciting discoveries about the STXBP1 epilepsy and how - get this: it appears that we need to use molecules to enhance the activity of excitatory synapses (rather than chill them out)!
As always, don’t forget to like, comment and subscribe because . Your comment and like will help spread awareness and understanding of the epilepsies around the world.
Now, onto our star of the week, Jean-François Perrier01:05 Jean-François Perrier
I'm based in Denmark but I was born in France. I am French and Danish now. I moved to Denmark about 28 years ago, first as a postdoc student and then after some years I became associate professor at the University of Copenhagen in the department of neuroscience, where I'm still working.01:23 Torie Robinson
So your research is largely to do with genetic epilepsy?01:29 Jean-François Perrier
Yes, actually I am new in the field. I've been working on fundamental research to try to understand how the brain is working for many, many years. And then about four years ago, I was contacted by a colleague who is very interested in one particular form of epilepsy. And he asked me if I could join because we work on slice preparation with a tool called electrophysiology - which means that we can record the activity of individual neurons - and then he believed that we could bring some novelty in the field by studying a model of the disease. That's what we did four years ago and for me, it was completely new. At the first meeting, I understood nothing they were saying, but then after some years working very hard with my postdoc and P.h.D students, we finally understood some important things.02:20 Torie Robinson
So the specific genetic epilepsy that you're working on, what's that called, please?02:25 Jean-François Perrier
So, it's called STXBP1. So STXBP1 is the name of the gene that is mutated in the patient that suffers from this form of epilepsy, actually. And it's a very important gene for communication between neurons. So, probably, you know that neurons communicate with each other by means of synapses. So when a synapse is active, it can release a small molecule that we call the neurotransmitter that communicates between the first neuron and the second neuron. And in terms of neurons, either to be more active or less active - depending on the synapse that is excitatory or inhibitory. So, the gene we are working on is important for all synapses - If it was not there, then the... If it was not expressed, then synapses... synaptic transmission wouldn't work, actually.03:16 Torie Robinson
Ha!03:17 Jean-François Perrier
So in the patients, there is what we call hyperinsufficiency. So, it means that the gene is expressed, but not enough to secure good communication. So, the communication between neurons is altered. Is impaired, actually.03:30 Torie Robinson
And I believe it can be an issue if the activity is overactive (which I think is more commonly understood by laypeople), but also it can be an issue if it's underactive as well?03:39 Jean-François Perrier
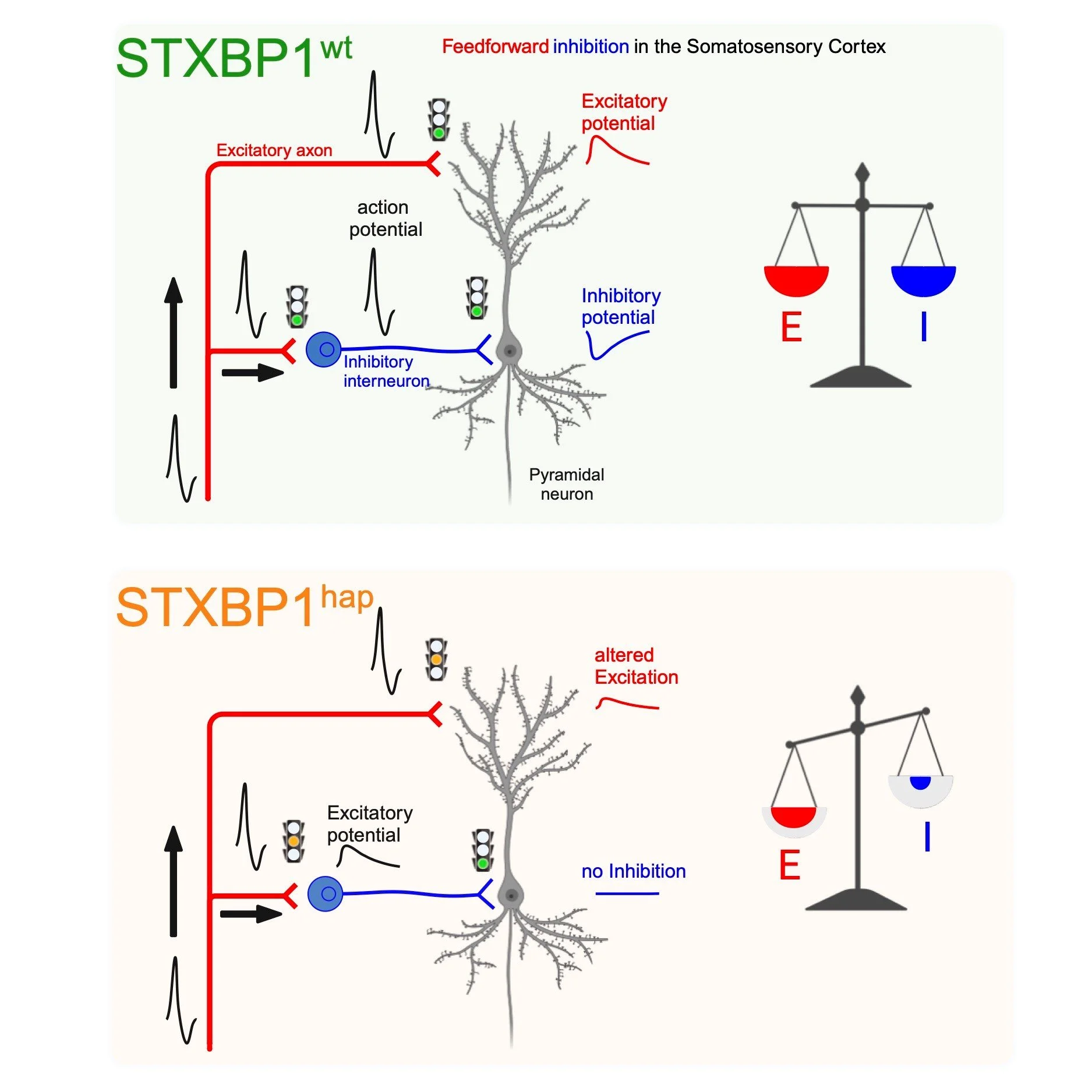
Yes, so a common reason for epilepsy is lack of inhibition. If there is not enough inhibition, then there is some hyperactivity in some area of the brain, and then it can be sufficient to trigger seizures, actually. So, that's a common rule for that. So, the challenge for us was to understand how a gene that is important for both excitatory and inhibitory synaptic transmission can lead to epilepsy, because if you impair both you would predict that the net activity of the brain should simply be decreased everywhere. But it's not the case because we know that all the patients suffer from epilepsy, actually. So, there is somehow a lack of inhibition, but we couldn't understand why. And that's where we started: we wanted to understand why there is such a lack of inhibition actually.04:29 Torie Robinson
Yeah, this is quite interesting, isn't it, because in lots of rare epilepsies - rare genetic epilepsies - not everybody will experience the seizures, right? So we'll commonly say it's a rare epilepsy, but actually lots of people with whatever mutation it is don't experience seizures. But you say with this mutation, every single person experiences..04:48 Jean-François Perrier
When I say everyone, it's about 90% of patients who suffer from very severe seizures, almost every day actually.04:57 Torie Robinson
And do they experience symptoms other than seizures too? Things like intellectual disability, autism, et cetera?05:03 Jean-François Perrier
They have very severe symptoms, epilepsy is one of them, it's the most common one, but they also suffer from intellectual disability, motor impairment, global delays, and autism in about 20% of the cases actually. So, it's a very, very severe disease actually and the problem is that now there is no good treatment. Clinicians try to reduce symptoms by trying different drugs that are available on the market, but, but there is no very good rationale for treating patients. So, things are tried and sometimes it helps, sometimes it doesn't. So, I think any improvement, any understanding could maybe help some patients actually. And that's what we are working on now.05:45 Torie Robinson
So, you've gone from in vitro to in vivo and that your principle does actually reduce seizures generally in these patients, is that correct?05:55 Jean-François Perrier
During three years, we have been working very hard on an in vitro preparation. So it means that we have an animal model that suffers from the same mutation found in patients. And what we do is that we make a slice preparation from the brain of these mice, and then we can record the activity of individual neurons and then study synapses (isolated synapses actually) one by one. And what we have been working on is the cortex where epilepsy is occurring in these patients. And we have been studying microcircuits of the brain called feedforward Inhibition. And it sounds complicated, but it's important if you want to understand what is going on actually. So, in this microcircuit, neurons receive excitatory input, so with excitatory synapse, but in parallel, they also receive inhibitory input because the excitatory input activates inhibitory interneurons that inhibit the principal neurons. So, in this way, with this microcircuit, there is an automatic balance between excitation and inhibition because every time the neuron is excited, it's inhibited at the same time, actually. And it's known that this microcircuit is essential for normal brain function. So, we have been studying that in our mouse model of the disease and what we found - and it was really unexpected - is that excitatory synapses were much more effective than inhibitory synapses! Inhibitory synapses were working normally and this was very surprising because I just told you that there is a lack of inhibition in patients! So, in fact what is happening is that the excitatory synapses are not able to activate the inhibitory interneuron and for this reason they remain silent, even though they could work properly. So we have a lack of excitation that paradoxically favours excitation. There is not enough inhibition because excitation is not working enough. And we think it's very important because it opens for a new principle for treating patients. We think that instead of treating inhibitory synapses - which is a common way to treat epilepsy; there are many drugs that target inhibitory synapses. We think instead for this particular disease, we should target excitatory synapses. And, it may sound weird, but we think that we should enhance the activity of excitatory synapses! And we have potential molecules that can do that, because there is a family of molecules that are called Positive Allosteric Modulators that increase the activity of excitatory synapses! So, the good thing is that they do not activate these synapses on themselves - because this would be very dangerous: it could trigger epilepsy. But they just bind to the synapse and if these synapses become active because the brain wants to activate them then the response is slightly enhanced, actually. We have tested our ID on our slice preparation and we got interesting results that we published in a paper, so it's very promising. And of course, the next step is to go to individuals because there is a huge gap between in vitro preparation to whole animal. And for this, we have established a model where we can record the epileptic activity that is occurring spontaneously in these animals (I mean, several times per hour). And then we can test the effect of the molecule I told you about (this Positive Allosteric Modulator) for AMPA receptors.
And our preliminary results - which we have not published yet - are very encouraging! I mean, we have tried different molecules and all of them induce a very strong dramatic decrease in the frequency of epileptic seizures in animals! So, we are very excited and we think that this result could be very interesting and important for patients.10:00 Torie Robinson
Well yes, important for patients obviously, number one, but secondarily for whole families…10:05 Jean-François Perrier
Yes.10:05 Torie Robinson
…because epilepsy in general affects a whole family, but the rare genetic, very, very severe epilepsies affect families even more. So, I imagine, should this be effective, fingers crossed, this will impact, positively impact, the lives of whole families!10:22 Jean-François Perrier
This is our hope. Of course, I don't want to promise anything, I mean, you know, there is always a gap between animal models and patients. But at least we, right now, we have all the reason to believe that this should be tested. And for this reason, we are in contact with different clinicians, both from the Filadelfia Hospital in Denmark and also with a clinician from Amsterdam University Hospital, and they express their interest in our findings and we hope that we will be able to start clinical trial in collaboration with them early this year or next year actually.10:58 Torie Robinson
That's very exciting and I often mention this in the podcast but we have to sometimes think about money, right? And it is funding that enables research to occur and trials to occur so I understand that you're just kind of in that area at the moment just waiting to see if grants come through and so that hopefully this can go ahead, is that correct?11:19 Jean-François Perrier
That's perfectly correct. I mean, if we want to go to clinical trial level, we need money because it's very expensive. And luckily, in Denmark, there are very generous foundations, private foundations, that have grants that we can apply to. And we just applied to two of the main foundations and we hope that they will share our enthusiasm and then fund our research. But we don't know yet. But we hope. If they don't do it, then we will just apply again and again because we all believe - I mean, when I say “we” it's my lab and the clinician - we all believe that it's a very interesting idea with a huge potential. So, we will keep applying until we get funded, actually.12:01 Torie Robinson
This is brilliant stuff. And for those who maybe think “Oh, well, these rare epilepsies, they don't necessarily affect anybody I know or any of my patients”, I think it's worth noting that, well, one of them at least will at some point and in addition - I'm not sure if you would agree with this (!) - but the research that you're carrying out and the trials that will come about actually will long-term benefit people who are affected by different types of epilepsies as well!12:26 Jean-François Perrier
This is our hope actually! So, we are already thinking about that actually! So, the disease we work on is called STXBP1 Encephalopathy and what we found is that it affects the activity of a particular type of interneuron in the context called the basket cells. But in fact, there are other types of epilepsy which also affect this neuron. For example: absence epilepsy or another form of epilepsy called Dravet Syndrome is also affecting the firing of these cells. So, we want to test, when we have time, if our principle can also benefit for this type of epilepsy.13:07 Torie Robinson
A huge thanks to Jean-François for sharing such surprising and exciting research results with us regarding his research into the rare genetic epilepsy STXBP1! You can find links to Jean-François and his work via the website, please don’t forget to like, comment, and subscribe, and see you next time! -
Jean-François Perrier
-
Jean-François Perrier is an Assistant Professor in XXX at the University of Copenhagen, Denmark.
Specialties: Neuroscience, neurophysiology, in vitro electrophysiology, Patch clamp recording
-